About the good and bad on ultra-fast charging
Ultra-fast Chargers
Nowhere is ultra-fast charging in bigger demand than with the electric vehicle. Recharging an EV in minutes replicates the convenience of filling 50 liters (13 gallons) of fuel into a tank that delivers 600kWh of energy. Such large energy storage in an electrochemical device is not practical as a battery with such a capacity would weigh 6 tons. Most Li-ion only produces about 150Wh per kg; the energy from fossil fuel is roughly 100 times higher. (See BU-1007: Net Calorific Value).
Charging an EV will always take longer than filling a tank, and the battery will always deliver less energy per weight than fossil fuel. Breaking the rule of law and forcing ultra-fast charging adds stress, even if the battery is designed for such a purpose. We must keep in mind that a battery is sluggish in nature. Like an aging man, its physical condition becomes less ideal with use and age. So is the ability to fast-charge.
Whether it’s an EV, e-bike, a flying object, a portable device or a hobby gadget, the following conditions must be respected when charging a battery ultra-fast:
- The battery must be designed to accept an ultra-fast charge and must be in good condition.
- Ultra-fast charging only applies during the first charge phase. The charge current should be lowered after the battery reaches 70 percent state-of-charge (SoC).
- All cells in the pack must be balanced and have ultra-low resistance. Aging cells often diverge in capacity and resistance, causing mismatch and undue stress on weaker cells.
- Ultra-fast charging can only be done under moderate temperatures, as low temperature slows the chemical reaction. Unused energy turns into gassing, metal-plating and heat.
A well-designed ultra-fast charger evaluates the condition of the “chemical battery” and makes adjustments according to the ability to receive charge. The charger should also include temperature compensations and other safety features to lower the charge current when certain conditions exist and halt the charge if the battery is under undue stress.
A “smart” battery running on SMBus or other protocols is responsible for the charge current. The system observes the battery condition and lowers or discontinues the charge if an anomaly occurs. Common irregularities are cell imbalance or the need for calibration. Some “smart” batteries stop functioning if the error is not corrected.
The maximum charge current a Li-ion can accept is governed by cell design, and not the cathode material, as is commonly assumed. The goal is to avoid lithium-plating on the anode and to keep the temperature under control. A thin anode with high porosity and small graphite particles enables ultra-fast charging because of the large surface area. Power Cells can be charged and discharged at high currents, but the energy density is low. Energy Cells, in comparison, have a thicker anode and lower porosity and the charge rate should 1C or less. Some hybrid Cells in NCA (nickel-cobalt-aluminum) can be charged above 1C with only moderate stress.
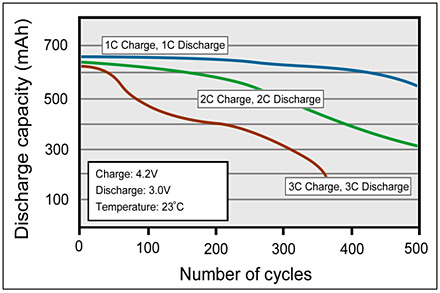
Apply the ultra-fast charge only when necessary. A well-designed ultra-fast charger should have charge-time selection to give the user the option to choose the least stressful charge for the time allotted. Figure 2 compares the cycle life of a typical lithium-ion battery when charged and discharged at 1C, 2C and 3C rates. The longevity can further be prolonged by charging and discharging below 1C; 0.8C is the recommended rate.
| Figure 2: Cycle performance of Li-ion with 1C, 2C and 3C charge and discharge. |
Summary
All batteries perform best at room temperature and with a moderate charge and discharge. Such a sheltered life style does not always reflect real world situations where a compact pack must be charged quickly and deliver high currents. Such typical applications are drones and remote control devices for hobbyist. Expect a short cycle life when a small pack must give all it has.
If fast charging and high load requirements are prerequisites, the rugged Power Cell is ideal; however, this increases battery size and weight. An analogy is choosing a heavy diesel engine to run a large truck instead of a souped-up engine designed for a sports car. The big diesel will outlive the light engine even if both have identical horsepower. Going heavier will be more economical in the long run. Table 3 summarizes the charge characteristics of lead, nickel and lithium-based batteries.
| Type | Chemistry | C rate | Time | Temperatures | Charge termination |
|---|---|---|---|---|---|
| Slow charger | NiCd Lead acid | 0.1C | 14h | 0ºC to 45ºC (32ºF to 113ºF) | Continuous low charge or fixed timer. Subject to overcharge. Remove battery when charged. |
| Rapid charger | NiCd, NiMH, Li-ion | 0.3-0.5C | 3-6h | 10ºC to 45ºC (50ºF to 113ºF) | Senses battery by voltage, current, temperature and time-out timer. |
| Fast charger | NiCd, NiMH, Li-ion | 1C | 1h+ | 10ºC to 45ºC (50ºF to 113ºF) | Same as a rapid charger with faster service. |
| Ultra-fast charger | Li-ion, NiCd, NiMH | 1-10C | 10-60 minutes | 10ºC to 45ºC (50ºF to 113ºF) | Applies ultra-fast charge to 70% SoC; limited to specialty batteries. |
Table 3: Charger characteristics. Each chemistry uses a unique charge termination.
Simple Guidelines Regarding Chargers
- If possible, charge at a moderate rate. Ultra-fast charging always causes stress.
- Fast and ultra-fast charge fills the battery only partially; a slower saturation charge completes the charge. Unlike lead acid, Li-ion does not need the saturation charge but the capacity will be a bit lower.
- Do not apply fast charge when the battery is cold or hot. only charge at moderate temperatures. Avoid fast charging an aged or low-performing battery.
'건강하고 행복하게 > 자동차생활' 카테고리의 다른 글
| ★★ Economical Driving (0) | 2017.02.16 |
|---|---|
| ★ What's the "Eco button"? (0) | 2017.02.16 |
| One charger and Two batteries (0) | 2017.02.09 |
| Absorbent Glass Mat (AGM) (0) | 2017.02.05 |
| ★ NOCO Genius Smart Battery Charger G3500, 7200 (0) | 2017.02.04 |