Metabolism
Metabolism
From Wikipedia, the free encyclopedia
Metabolism is the set of chemical reactions that occur in living organisms in order to maintain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories. Catabolism breaks down large molecules, for example to harvest energy in cellular respiration. Anabolism, on the other hand, uses energy to construct components of cells such as proteins and nucleic acids.
The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed into another by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable but thermodynamically unfavorable reactions by coupling them to favorable ones. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or signals from other cells.
The metabolism of an organism determines which substances it will find nutritious and which it will find poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals.[1] The speed of metabolism, the metabolic rate, also influences how much food an organism will require.
A striking feature of metabolism is the similarity of the basic metabolic pathways between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all organisms, being found in species as diverse as the unicellular bacteria Escherichia coli and huge multicellular organisms like elephants.[2] These striking similarities in metabolism are most likely the result of the high efficiency of these pathways, and of their early appearance in evolutionary history.[3][4]
Contents[hide] |
[edit] Key biochemicals
- Further information: Biomolecule, cell (biology) and biochemistry
Most of the structures that make up animals, plants and microbes are made from three basic classes of molecule: amino acids, carbohydrates and lipids (often called fats). As these molecules are vital for life, metabolism focuses on making these molecules, in the construction of cells and tissues, or breaking them down and using them as a source of energy, in the digestion and use of food. Many important biochemicals can be joined together to make polymers such as DNA and proteins. These macromolecules are essential parts of all living organisms. Some of the most common biological polymers are listed in the table below.
| Type of molecule | Name of monomer forms | Name of polymer forms | Examples of polymer forms |
|---|---|---|---|
| Amino acids | Amino acids | Proteins (also called polypeptides) | Fibrous proteins and globular proteins |
| Carbohydrates | Monosaccharides | Polysaccharides | Starch, glycogen and cellulose |
| Nucleic acids | Nucleotides | Polynucleotides | DNA and RNA |
[edit] Amino acids and proteins
Proteins are made of amino acids arranged in a linear chain and joined together by peptide bonds. Many proteins are the enzymes that catalyze the chemical reactions in metabolism. Other proteins have structural or mechanical functions, such as the proteins that form the cytoskeleton, a system of scaffolding that maintains the cell shape.[5] Proteins are also important in cell signaling, immune responses, cell adhesion, active transport across membranes and the cell cycle.[6]
[edit] Lipids
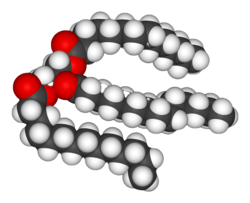
Lipids are the most diverse group of biochemicals. Their main structural uses are as part of biological membranes such as the cell membrane, or as a source of energy.[6] Lipids are usually defined as hydrophobic or amphipathic biological molecules that will dissolve in organic solvents such as benzene or chloroform.[7] The fats are a large group of compounds that contain fatty acids and glycerol; a glycerol molecule attached to three fatty acid esters is a triacylglyceride.[8] Several variations on this basic structure exist, including alternate backbones such as sphingosine in the sphingolipids, and hydrophilic groups such as phosphate in phospholipids. Steroids such as cholesterol are another major class of lipids that are made in cells.[9]
[edit] Carbohydrates
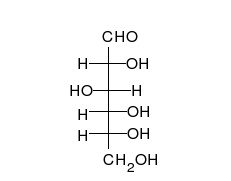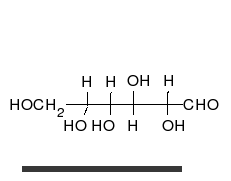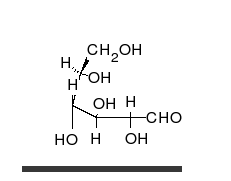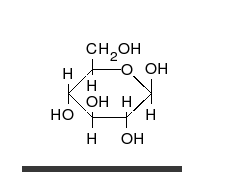
Carbohydrates are straight-chain aldehydes or ketones with many hydroxyl groups that can exist as straight chains or rings. Carbohydrates are the most abundant biological molecules, and fill numerous roles, such as the storage and transport of energy (starch, glycogen) and structural components (cellulose in plants, chitin in animals).[6] The basic carbohydrate units are called monosaccharides and include galactose, fructose, and most importantly glucose. Monosaccharides can be linked together to form polysaccharides in almost limitless ways.[10]
[edit] Nucleotides
The polymers DNA and RNA are long chains of nucleotides. These molecules are critical for the storage and use of genetic information, through the processes of transcription and protein biosynthesis.[6] This information is protected by DNA repair mechanisms and propagated through DNA replication. A few viruses have an RNA genome, for example HIV, which uses reverse transcription to create a DNA template from its viral RNA genome.[11] RNA in ribozymes such as spliceosomes and ribosomes is similar to enzymes as it can catalyze chemical reactions. Individual nucleosides are made by attaching a nucleobase to a ribose sugar. These bases are heterocyclic rings containing nitrogen, classified as purines or pyrimidines. Nucleotides also act as coenzymes in metabolic group transfer reactions.[12]
[edit] Coenzymes

- Further information: Coenzyme
Metabolism involves a vast array of chemical reactions, but most fall under a few basic types of reactions that involve the transfer of functional groups.[13] This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions.[12] These group-transfer intermediates are called coenzymes. Each class of group-transfer reaction is carried out by a particular coenzyme, which is the substrate for a set of enzymes that produce it, and a set of enzymes that consume it. These coenzymes are therefore continuously being made, consumed and then recycled.[14]
One central coenzyme is adenosine triphosphate (ATP), the universal energy currency of cells. This nucleotide is used to transfer chemical energy between different chemical reactions. There is only a small amount of ATP in cells, but as it is continuously regenerated, the human body can use about its own weight in ATP per day.[14] ATP acts as a bridge between catabolism and anabolism, with catabolic reactions generating ATP and anabolic reactions consuming it. It also serves as a carrier of phosphate groups in phosphorylation reactions.
A vitamin is an organic compound needed in small quantities that cannot be made in the cells. In human nutrition, most vitamins function as coenzymes after modification; for example, all water-soluble vitamins are phosphorylated or are coupled to nucleotides when they are used in cells.[15] Nicotinamide adenine dinucleotide (NADH), a derivative of vitamin B3 (niacin), is an important coenzyme that acts as a hydrogen acceptor. Hundreds of separate types of dehydrogenases remove electrons from their substrates and reduce NAD+ into NADH. This reduced form of the coenzyme is then a substrate for any of the reductases in the cell that need to reduce their substrates.[16] Nicotinamide adenine dinucleotide exists in two related forms in the cell, NADH and NADPH. The NAD+/NADH form is more important in catabolic reactions, while NADP+/NADPH is used in anabolic reactions.
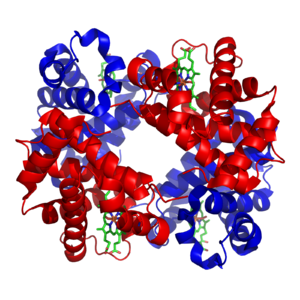
[edit] Minerals and cofactors
- Further information: Physiology, bioinorganic chemistry, cofactor and iron metabolism
Inorganic elements play critical roles in metabolism; some are abundant (e.g. sodium and potassium) while others function at minute concentrations. About 99% of mammals' mass are the elements carbon, nitrogen, calcium, sodium, chlorine, potassium, hydrogen, phosphorus, oxygen and sulfur.[17] The organic compounds (proteins, lipids and carbohydrates) contain the majority of the carbon and nitrogen and most of the oxygen and hydrogen is present as water.[17]
The abundant inorganic elements act as ionic electrolytes. The most important ions are sodium, potassium, calcium, magnesium, chloride, phosphate, and the organic ion bicarbonate. The maintenance of precise gradients across cell membranes maintains osmotic pressure and pH.[18] Ions are also critical for nerves and muscles, as action potentials in these tissues are produced by the exchange of electrolytes between the extracellular fluid and the cytosol.[19] Electrolytes enter and leave cells through proteins in the cell membrane called ion channels. For example, muscle contraction depends upon the movement of calcium, sodium and potassium through ion channels in the cell membrane and T-tubules.[20]
The transition metals are usually present as trace elements in organisms, with zinc and iron being most abundant.[21][22] These metals are used in some proteins as cofactors and are essential for the activity of enzymes such as catalase and oxygen-carrier proteins such as hemoglobin.[23] These cofactors are bound tightly to a specific protein; although enzyme cofactors can be modified during catalysis, cofactors always return to their original state after catalysis has taken place. The metal micronutrients are taken up into organisms by specific transporters and bound to storage proteins such as ferritin or metallothionein when not being used.[24][25]
1. Catabolism
- Further information: Catabolism
Catabolism is the set of metabolic processes that break down large molecules. These include breaking down and oxidising food molecules. The purpose of the catabolic reactions is to provide the energy and components needed by anabolic reactions. The exact nature of these catabolic reactions differ from organism to organism, with organic molecules being used as a source of energy in organotrophs, while lithotrophs use inorganic substrates and phototrophs capture sunlight as chemical energy. However, all these different forms of metabolism depend on redox reactions that involve the transfer of electrons from reduced donor molecules such as organic molecules, water, ammonia, hydrogen sulfide or ferrous ions to acceptor molecules such as oxygen, nitrate or sulfate.[26] In animals these reactions involve complex organic molecules being broken down to simpler molecules, such as carbon dioxide and water. In photosynthetic organisms such as plants and cyanobacteria, these electron-transfer reactions do not release energy, but are used as a way of storing energy absorbed from sunlight.[6]
The most common set of catabolic reactions in animals can be separated into three main stages. In the first, large organic molecules such as proteins, polysaccharides or lipids are digested into their smaller components outside cells. Next, these smaller molecules are taken up by cells and converted to yet smaller molecules, usually acetyl coenzyme A (CoA), which releases some energy. Finally, the acetyl group on the CoA is oxidised to water and carbon dioxide in the citric acid cycle and electron transport chain, releasing the energy that is stored by reducing the coenzyme nicotinamide adenine dinucleotide (NAD+) into NADH.
[edit] Digestion
- Further information: Digestion and gastrointestinal tract
Macromolecules such as starch, cellulose or proteins cannot be rapidly taken up by cells and need to be broken into their smaller units before they can be used in cell metabolism. Several common classes of enzymes digest these polymers. These digestive enzymes include proteases that digest proteins into amino acids, as well as glycoside hydrolases that digest polysaccharides into monosaccharides.
Microbes simply secrete digestive enzymes into their surroundings,[27][28] while animals only secrete these enzymes from specialized cells in their guts.[29] The amino acids or sugars released by these extracellular enzymes are then pumped into cells by specific active transport proteins.[30][31]
[edit] Energy from organic compounds
- Further information: Cellular respiration, fermentation, carbohydrate catabolism, fat catabolism and protein catabolism
Carbohydrate catabolism is the breakdown of carbohydrates into smaller units. Carbohydrates are usually taken into cells once they have been digested into monosaccharides.[32] once inside, the major route of breakdown is glycolysis, where sugars such as glucose and fructose are converted into pyruvate and some ATP is generated.[33] Pyruvate is an intermediate in several metabolic pathways, but the majority is converted to acetyl-CoA and fed into the citric acid cycle. Although some more ATP is generated in the citric acid cycle, the most important product is NADH, which is made from NAD+ as the acetyl-CoA is oxidized. This oxidation releases carbon dioxide as a waste product. In anaerobic conditions, glycolysis produces lactate, through the enzyme lactate dehydrogenase re-oxidizing NADH to NAD+ for re-use in glycolysis. An alternative route for glucose breakdown is the pentose phosphate pathway, which reduces the coenzyme NADPH and produces pentose sugars such as ribose, the sugar component of nucleic acids.
Fats are catabolised by hydrolysis to free fatty acids and glycerol. The glycerol enters glycolysis and the fatty acids are broken down by beta oxidation to release acetyl-CoA, which then is fed into the citric acid cycle. Fatty acids release more energy upon oxidation than carbohydrates because carbohydrates contain more oxygen in their structures.
Amino acids are either used to synthesize proteins and other biomolecules, or oxidized to urea and carbon dioxide as a source of energy.[34] The oxidation pathway starts with the removal of the amino group by a transaminase. The amino group is fed into the urea cycle, leaving a deaminated carbon skeleton in the form of a keto acid. Several of these keto acids are intermediates in the citric acid cycle, for example the deamination of glutamate forms α-ketoglutarate.[35] The glucogenic amino acids can also be converted into glucose, through gluconeogenesis (discussed below).[36]
[edit] Energy transformations
[edit] Oxidative phosphorylation

- Further information: Oxidative phosphorylation, chemiosmosis and mitochondrion
In oxidative phosphorylation, the electrons removed from food molecules in pathways such as the citric acid cycle are transferred to oxygen and the energy released used to make ATP. This is done in eukaryotes by a series of proteins in the membranes of mitochondria called the electron transport chain. In prokaryotes, these proteins are found in the cell's inner membrane.[37] These proteins use the energy released from passing electrons from reduced molecules like NADH onto oxygen to pump protons across a membrane.[38]
Pumping protons out of the mitochondria creates a proton concentration difference across the membrane and generates an electrochemical gradient.[39] This force drives protons back into the mitochondrion through the base of an enzyme called ATP synthase. The flow of protons makes the stalk subunit rotate, causing the active site of the synthase domain to change shape and phosphorylate adenosine diphosphate - turning it into ATP.[14]
[edit] Energy from inorganic compounds
- Further information: Microbial metabolism and nitrogen cycle
Chemolithotrophy is a type of metabolism found in prokaryotes where energy is obtained from the oxidation of inorganic compounds. These organisms can use hydrogen,[40] reduced sulfur compounds (such as sulfide, hydrogen sulfide and thiosulfate),[41] ferrous iron (FeII)[42] or ammonia[43] as sources of reducing power and they gain energy from the oxidation of these compounds with electron acceptors such as oxygen or nitrite.[44] These microbial processes are important in global biogeochemical cycles such as acetogenesis, nitrification and denitrification and are critical for soil fertility.[45][46]
[edit] Energy from light
- Further information: Phototroph, photophosphorylation, chloroplast
The energy in sunlight is captured by plants, cyanobacteria, purple bacteria, green sulfur bacteria and some protists. This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below. The energy capture and carbon fixation systems can however operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds.[47][48]
The capture of solar energy is a process that is similar in principle to oxidative phosphorylation, as it involves energy being stored as a proton concentration gradient and this proton motive force then driving ATP synthesis.[14] The electrons needed to drive this electron transport chain come from light-gathering proteins called photosynthetic reaction centres. These structures are classed into two types depending on the type of photosynthetic pigment present, with most photosynthetic bacteria only having one type of reaction center, while plants and cyanobacteria have two.[49]
In plants, photosystem II uses light energy to remove electrons from water, releasing oxygen as a waste product. The electrons then flow to the cytochrome b6f complex, which uses their energy to pump protons across the thylakoid membrane in the chloroplast.[50] These protons move back through the membrane as they drive the ATP synthase, as before. The electrons then flow through photosystem I and can then either be used to reduce the coenzyme NADP+, for use in the Calvin cycle which is discussed below, or recycled for further ATP generation.[51]
2. Anabolism
- Further information: Anabolism
Anabolism is the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from small and simple precursors. Anabolism involves three basic stages. Firstly, the production of precursors such as amino acids, monosaccharides, isoprenoids and nucleotides, secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such as proteins, polysaccharides, lipids and nucleic acids.
Organisms differ in how many of the molecules in their cells they can construct for themselves. Autotrophs such as plants can construct the complex organic molecules in cells such as polysaccharides and proteins from simple molecules like carbon dioxide and water. Heterotrophs, on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from inorganic oxidation reactions.
[edit] Carbon fixation
- Further information: Photosynthesis, carbon fixation and chemosynthesis
Photosynthesis is the synthesis of carbohydrates from sunlight, carbon dioxide (CO2) and water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by the photosynthetic reaction centres, as described above, to convert CO2 into glycerate 3-phosphate, which can then be converted into glucose. This carbon-fixation reaction is carried out by the enzyme RuBisCO as part of the Calvin – Benson cycle.[52] Three types of photosynthesis occur in plants, C3 carbon fixation, C4 carbon fixation and CAM photosynthesis. These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO2 directly, while C4 and CAM photosynthesis incorporate the CO2 into other compounds first, as adaptations to deal with intense sunlight and dry conditions.[53]
In photosynthetic prokaryotes the mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin – Benson cycle, a reversed citric acid cycle,[54] or the carboxylation of acetyl-CoA.[55][56] Prokaryotic chemoautotrophs also fix CO2 through the Calvin – Benson cycle, but use energy from inorganic compounds to drive the reaction.[57]
[edit] Carbohydrates and glycans
- Further information: Gluconeogenesis, glyoxylate cycle, glycogenesis and glycosylation
In carbohydrate anabolism, simple organic acids can be converted into monosaccharides such as glucose and then used to assemble polysaccharides such as starch. The generation of glucose from compounds like pyruvate, lactate, glycerol, glycerate 3-phosphate and amino acids is called gluconeogenesis. Gluconeogenesis converts pyruvate to glucose-6-phosphate through a series of intermediates, many of which are shared with glycolysis.[33] However, this pathway is not simply glycolysis run in reverse, as several steps are catalyzed by non-glycolytic enzymes. This is important as it allows the formation and breakdown of glucose to be regulated separately and prevents both pathways from running simultaneously in a futile cycle.[58][59]
Although fat is a common way of storing energy, in vertebrates such as humans the fatty acids in these stores cannot be converted to glucose through gluconeogenesis as these organisms cannot convert acetyl-CoA into pyruvate.[60] As a result, after long-term starvation, vertebrates need to produce ketone bodies from fatty acids to replace glucose in tissues such as the brain that cannot metabolize fatty acids.[61] In other organisms such as plants and bacteria, this metabolic problem is solved using the glyoxylate cycle, which bypasses the decarboxylation step in the citric acid cycle and allows the transformation of acetyl-CoA to oxaloacetate, where it can be used for the production of glucose.[62][60]
Polysaccharides and glycans are made by the sequential addition of monosaccharides by glycosyltransferase from a reactive sugar-phosphate donor such as uridine diphosphate glucose (UDP-glucose) to an acceptor hydroxyl group on the growing polysaccharide. As any of the hydroxyl groups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures.[63] The polysaccharides produced can have structural or metabolic functions themselves, or be transferred to lipids and proteins by enzymes called oligosaccharyltransferases.[64][65]
[edit] Fatty acids, isoprenoids and steroids
- Further information: Fatty acid synthesis, steroid metabolism

Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the actyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional type I protein,[66] while in plant plastids and bacteria separate type II enzymes perform each step in the pathway.[67][68]
Terpenes and isoprenoids are a large class of lipids that include the carotenoids and form the largest class of plant natural products.[69] These compounds are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate.[70] These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA,[71] while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates.[72][70] one important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol.[73] Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.[74][73]
[edit] Proteins
- Further information: Protein biosynthesis, amino acid synthesis
Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all twenty, but mammals can synthesize only the ten nonessential amino acids.[6] Thus, the essential amino acids must be obtained from food. All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen is provided by glutamate and glutamine. Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid.[75]
Amino acids are made into proteins by being joined together in a chain by peptide bonds. Each different protein has a unique sequence of amino acid residues: this is its primary structure. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to a transfer RNA molecule through an ester bond. This aminoacyl-tRNA precursor is produced in an ATP-dependent reaction carried out by an aminoacyl tRNA synthetase.[76] This aminoacyl-tRNA is then a substrate for the ribosome, which joins the amino acid onto the elongating protein chain, using the sequence information in a messenger RNA.[77]
[edit] Nucleotide synthesis and salvage
- Further information: Nucleotide salvage, pyrimidine biosynthesis, and purine metabolism
Nucleotides are made from amino acids, carbon dioxide and formic acid in pathways that require large amounts of metabolic energy.[78] Consequently, most organisms have efficient systems to salvage preformed nucleotides.[78][79] Purines are synthesized as nucleosides (bases attached to ribose). Both adenine and guanine are made from the precursor nucleoside inosine monophosphate, which is synthesized using atoms from the amino acids glycine, glutamine, and aspartic acid, as well as formate transferred from the coenzyme tetrahydrofolate. Pyrimidines, on the other hand, are synthesized from the base orotate, which is formed from glutamine and aspartate.[80]
[edit] Xenobiotics and redox metabolism
- Further information: Xenobiotic metabolism, drug metabolism and antioxidants
All organisms are constantly exposed to compounds that they cannot use as foods and would be harmful if they accumulated in cells, as they have no metabolic function. These potentially damaging compounds are called xenobiotics.[81] Xenobiotics such as synthetic drugs, natural poisons and antibiotics are detoxified by a set of xenobiotic-metabolizing enzymes. In humans, these include cytochrome P450 oxidases,[82] UDP-glucuronosyltransferasess,[83] and glutathione S-transferases.[84] This system of enzymes acts in three stages to firstly oxidize the xenobiotic (phase I) and then conjugate water-soluble groups onto the molecule (phase II). The modified water-soluble xenobiotic can then be pumped out of cells and in multicellular organisms may be further metabolized before being excreted (phase III). In ecology, these reactions are particularly important in microbial biodegradation of pollutants and the bioremediation of contaminated land and oil spills.[85] Many of these microbial reactions are shared with multicellular organisms, but due to the incredible diversity of types of microbes these organisms are able to deal with a far wider range of xenobiotics than multicellular organisms, and can degrade even persistent organic pollutants such as organochloride compounds.[86]
A related problem for aerobic organisms is oxidative stress.[87] Here, processes including oxidative phosphorylation and the formation of disulfide bonds during protein folding produce reactive oxygen species such as hydrogen peroxide.[88] These damaging oxidants are removed by antioxidant metabolites such as glutathione and enzymes such as catalases and peroxidases.[89][90]
[edit] Thermodynamics of living organisms
- Further information: Biological thermodynamics
Living organisms must obey the laws of thermodynamics, which describe the transfer of heat and work. The second law of thermodynamics states that in any closed system, the amount of entropy (disorder) will tend to increase. Although living organisms' amazing complexity appears to contradict this law, life is possible as all organisms are open systems that exchange matter and energy with their surroundings. Thus living systems are not in equilibrium, but instead are dissipative systems that maintain their state of high complexity by causing a larger increase in the entropy of their environments.[91] The metabolism of a cell achieves this by coupling the spontaneous processes of catabolism to the non-spontaneous processes of anabolism. In thermodynamic terms, metabolism maintains order by creating disorder.[92]
[edit] Regulation and control
- Further information: Metabolic pathway, metabolic control analysis, hormone and cell signaling
As the environments of most organisms are constantly changing, the reactions of metabolism must be finely regulated to maintain a constant set of conditions within cells, a condition called homeostasis.[93][94] Metabolic regulation also allows organisms to respond to signals and interact actively with their environments.[95] Two closely-linked concepts are important for understanding how metabolic pathways are controlled. Firstly, the regulation of an enzyme in a pathway is how its activity is increased and decreased in response to signals. Secondly, the control exerted by this enzyme is the effect that these changes in its activity have on the overall rate of the pathway (the flux through the pathway).[96] For example, an enzyme may show large changes in activity (i.e. it is highly regulated) but if these changes have little effect on the flux of a metabolic pathway, then this enzyme is not involved in the control of the pathway.[97]

There are multiple levels of metabolic regulation. In intrinsic regulation, the metabolic pathway self-regulates to respond to changes in the levels of substrates or products; for example, a decrease in the amount of product can increase the flux through the pathway to compensate.[96] This type of regulation often involves allosteric regulation of the activities of multiple enzymes in the pathway.[98] Extrinsic control involves a cell in a multicellular organism changing its metabolism in response to signals from other cells. These signals are usually in the form of soluble messengers such as hormones and growth factors and are detected by specific receptors on the cell surface.[99] These signals are then transmitted inside the cell by second messenger systems that often involved the phosphorylation of proteins.[100]
A very well understood example of extrinsic control is the regulation of glucose metabolism by the hormone insulin.[101] Insulin is produced in response to rises in blood glucose levels. Binding of the hormone to insulin receptors on cells then activates a cascade of protein kinases that cause the cells to take up glucose and convert it into storage molecules such as fatty acids and glycogen.[102] The metabolism of glycogen is controlled by activity of phosphorylase, the enzyme that breaks down glycogen, and glycogen synthase, the enzyme that makes it. These enzymes are regulated in a reciprocal fashion, with phosphorylation inhibiting glycogen synthase, but activating phosphorylase. Insulin causes glycogen synthesis by activating protein phosphatases and producing a decrease in the phosphorylation of these enzymes.[103]
[edit] Evolution
- Further information: Molecular evolution and phylogenetics

The central pathways of metabolism described above, such as glycolysis and the citric acid cycle, are present in all three domains of living things and were present in the last universal ancestor.[104][2] This universal ancestral cell was prokaryotic and probably a methanogen that had extensive amino acid, nucleotide, carbohydrate and lipid metabolism.[105][106] The retention of these ancient pathways during later evolution may be the result of these reactions being an optimal solution to their particular metabolic problems, with pathways such as glycolysis and the citric acid cycle producing their end products highly efficiently and in a minimal number of steps.[3][4] The first pathways of enzyme-based metabolism may have been parts of purine nucleotide metabolism, with previous metabolic pathways being part of the ancient RNA world.[107]
Many models have been proposed to describe the mechanisms by which novel metabolic pathways evolve. These include the sequential addition of novel enzymes to a short ancestral pathway, the duplication and then divergence of entire pathways as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway.[108] The relative importance of these mechanisms is unclear, but genomic studies have shown that enzymes in a pathway are likely to have a shared ancestry, suggesting that many pathways have evolved in a step-by-step fashion with novel functions being created from pre-existing steps in the pathway.[109] An alternative model comes from studies that trace the evolution of proteins' structures in metabolic networks, this has suggested that enzymes are pervasively recruited, borrowing enzymes to perform similar functions in different metabolic pathways (evident in the MANET database)[110] These recruitment processes result in an evolutionary enzymatic mosaic.[111] A third possibility is that some parts of metabolism might exist as "modules" that can be reused in different pathways and perform similar functions on different molecules.[112]
As well as the evolution of new metabolic pathways, evolution can also cause the loss of metabolic functions. For example, in some parasites metabolic processes that are not essential for survival are lost and preformed amino acids, nucleotides and carbohydrates may instead be scavenged from the host.[113] Similar reduced metabolic capabilities are seen in endosymbiotic organisms.[114]
[edit] Investigation and manipulation
- Further information: Protein methods, proteomics, metabolomics and metabolic network modelling

Classically, metabolism is studied by a reductionist approach that focuses on a single metabolic pathway. Particularly valuable is the use of radioactive tracers at the whole-organism, tissue and cellular levels, which define the paths from precursors to final products by identifying radioactively-labelled intermediates and products.[115] The enzymes that catalyze these chemical reactions can then be purified and their kinetics and responses to inhibitors investigated. A parallel approach is to identify the small molecules in a cell or tissue; the complete set of these molecules is called the metabolome. Overall, these studies give a good view of the structure and function of simple metabolic pathways, but are inadequate when applied to more complex systems such as the metabolism of a complete cell.[116]
An idea of the complexity of the metabolic networks in cells that contain thousands of different enzymes is given by the figure showing the interactions between just 43 proteins and 40 metabolites to the right: the sequences of genomes provide lists containing anything up to 45,000 genes.[117] However, it is now possible to use this genomic data to reconstruct complete networks of biochemical reactions and produce more holistic mathematical models that may explain and predict their behavior.[118] These models are especially powerful when used to integrate the pathway and metabolite data obtained through classical methods with data on gene expression from proteomic and DNA microarray studies.[119]
A major technological application of this information is metabolic engineering. Here, organisms such as yeast, plants or bacteria are genetically-modified to make them more useful in biotechnology and aid the production of drugs such as antibiotics or industrial chemicals such as 1,3-propanediol and shikimic acid.[120] These genetic modifications usually aim to reduce the amount of energy used to produce the product, increase yields and reduce the production of wastes.[121]
[edit] History
- Further information: History of biochemistry and history of molecular biology

The term metabolism is derived from the Greek Μεταβολισμός – "Metabolismos" for "change", or "overthrow".[122] The history of the scientific study of metabolism spans several centuries and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry. The concept of metabolism dates back to Ibn al-Nafis (1213-1288), who stated that "the body and its parts are in a continuous state of dissolution and nourishment, so they are inevitably undergoing permanent change."[123] The first controlled experiments in human metabolism were published by Santorio Santorio in 1614 in his book Ars de statica medecina.[124] He described how he weighed himself before and after eating, sleeping, working, sex, fasting, drinking, and excreting. He found that most of the food he took in was lost through what he called "insensible perspiration".
In these early studies, the mechanisms of these metabolic processes had not been identified and a vital force was thought to animate living tissue.[125] In the 19th century, when studying the fermentation of sugar to alcohol by yeast, Louis Pasteur concluded that fermentation was catalyzed by substances within the yeast cells he called "ferments". He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells."[126] This discovery, along with the publication by Friedrich Wöhler in 1828 of the chemical synthesis of urea,[127] proved that the organic compounds and chemical reactions found in cells were no different in principle than any other part of chemistry.
It was the discovery of enzymes at the beginning of the 20th century by Eduard Buchner that separated the study of the chemical reactions of metabolism from the biological study of cells, and marked the beginnings of biochemistry.[128] The mass of biochemical knowledge grew rapidly throughout the early 20th century. one of the most prolific of these modern biochemists was Hans Krebs who made huge contributions to the study of metabolism.[129] He discovered the urea cycle and later, working with Hans Kornberg, the citric acid cycle and the glyoxylate cycle.[130][62] Modern biochemical research has been greatly aided by the development of new techniques such as chromatography, X-ray diffraction, NMR spectroscopy, radioisotopic labelling, electron microscopy and molecular dynamics simulations. These techniques have allowed the discovery and detailed analysis of the many molecules and metabolic pathways in cells
Basal metabolic rate
Basal metabolic rate (BMR) is the amount of energy expended while at rest in a neutrally temperate environment, in the post-absorptive state (meaning that the digestive system is inactive, which requires about twelve hours of fasting in humans). The release of energy in this state is sufficient only for the functioning of the vital organs, such as the heart, lungs, brain and the rest of the nervous system, liver, kidneys, sex organs, muscles and skin. BMR decreases with age and with the loss of lean body mass. Increased cardiovascular exercise and muscle mass can increase BMR. Illness, previously consumed food and beverages, environmental temperature, and stress levels can affect one's overall energy expenditure, and can affect one's BMR.
BMR is measured under very restrictive circumstances when a person is awake, but at complete rest. An accurate BMR measurement requires that the person's sympathetic nervous system is not stimulated. A more common and closely related measurement, used under less strict conditions, is resting metabolic rate (RMR).[1]
BMR and RMR are measured by gas analysis through either direct or indirect calorimetry, though a rough estimation can be acquired through an equation using age, sex, height, and weight. Studies of energy metabolism using both methods provide convincing evidence for the validity of the respiratory quotient (R.Q.), which measures the inherent composition and utilization of carbohydrates, fats and proteins as they are converted to energy substrate units that can be used by the body as energy.
Contents
[hide]
[edit] Nutrition and dietary considerations
Basal metabolic rate is usually by far the largest component of total caloric expenditure. However, the Harris-Benedict equations are only approximate and variation in BMR (reflecting varying body composition), in physical activity levels, and in energy expended in thermogenesis make it difficult to estimate the dietary consumption any particular individual needs in order to maintain body weight. 2000 kilocalories is often quoted but is no more than a guideline.
[edit] Physiology
Both basal metabolic rate and resting metabolic rate are usually expressed in terms of daily rates of energy expenditure. The early work of the scientists J. Arthur Harris and Francis G. Benedict showed that approximate values could be derived using body surface area (computed from height and weight), age, and sex, along with the oxygen and carbon dioxide measures taken from calorimetry. Studies also showed that by eliminating the sex differences that occur with the accumulation of adipose tissue by expressing metabolic rate per unit of "fat-free" or lean body weight, the values between sexes for basal metabolism are essentially the same. Exercise physiology textbooks have tables to show the conversion of height and body surface area as they relate to weight and basal metabolic values.
The primary organ responsible for regulating metabolism is the hypothalamus. The hypothalamus is located on the brain stem and forms the floor and part of the lateral walls of the third ventricle of the cerebrum. The chief functions of the hypothalamus are:
-
- control and integration of activities of the autonomic nervous system (ANS)
- The ANS regulates contraction of smooth muscle and cardiac muscle, along with secretions of many endocrine organs such as the thyroid gland (associated with many metabolic disorders).
- Through the ANS, the hypothalamus is the main regulator of visceral activities, such as heart rate, movement of food through the gastrointestinal tract, and contraction of the urinary bladder.
- production and regulation of feelings of rage and aggression
- regulation of body temperature
- regulation of food intake, through two centers:
- The feeding center or hunger center is responsible for the sensations that cause us to seek food. When sufficient food or substrates have been received and leptin is high, then the satiety center is stimulated and sends impulses that inhibit the feeding center. When insufficient food is present in the stomach and ghrelin levels are high, receptors in the hypothalamus initiate the sense of hunger.
- The thirst center operates similarly when certain cells in the hypothalamus are stimulated by the rising osmotic pressure of the extracellular fluid. If thirst is satisfied, osmotic pressure decreases.
All of these functions taken together form a survival mechanism that causes us to sustain the body processes that BMR and RMR measure.
[edit] BMR estimation formulas
Several prediction equations exist. Historically most notable was Harris-Benedict equation, which was created in 1919.
The original equations from Harris and Benedict are:
-
- for men,

- for women,
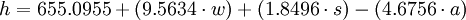
where h = total heat production per 24 hours at complete rest in kilocalories (kcal), w = weight in kilograms, s = stature (height) in centimeters, and a = age in years, and with the difference in BMR for men and women being mainly due to differences in body weight.[2]
It was the best prediction equation until recently, when MD Mifflin and ST St Jeor in 1990 created new equation:
-

During last 100 years lifestyle changed and a survey in 2005 showed it to be about 5% more accurate.
[edit] Example calculation
For example, a 55 year old woman weighing 130 lb (59 kg) and 5 feet 6 inches (168 cm) tall would have a BMR of 1266 kcal per day or 52.8 kcal/h (61.3 watts). This is computed by as 655 + (9.6 x 59) + (1.8 x 168) - (4.7 x 55).
To calculate Daily Calorie Needs, this BMR value is multiplied by a factor with a value between 1.2 and 1.9, depending on the person's activity level.
An online BMR calculator for both metric and non-metric values can be found at this link [1]
[edit] Animal BMR
Kleiber's law relates the BMR for animals of different sizes and the observations indicate that the BMR is proportional to the 3/4 power of body mass. Warm blooded, cold blooded and unicellular animals fit on different curves.
[edit] Biochemistry
Energy expenditure breakdown
liver
27%
brain
19%
heart
7%
kidneys
10%
skeletal muscle
18%
other organs
19%
About 70% of a human's total energy expenditure is due to the basal life processes within the organs of the body (see table). About 20% of one's energy expenditure comes from physical activity and another 10% from thermogenesis, or digestion of food.[citation needed] All of these processes require an intake of oxygen along with coenzymes to provide energy for survival (usually from macronutrients like carbohydrates, fats, and proteins) and expel carbon dioxide, which is explained by the Krebs cycle.
What enables the Krebs cycle to perform metabolic changes to fats, carbohydrates, and proteins is energy which can be defined as the ability or capacity to do work. The breakdown of large molecules into smaller molecules associated with release of energy is catabolism. The building up process is termed anabolism. The breakdown of proteins into amino acids is an example of catabolism while the formation of proteins from amino acids is an anabolic process.
Exergonic reactions are energy-releasing reactions and are generally catabolic. Endergonic reactions require energy and include anabolic reactions and the contraction of muscle. Metabolism is the total of all catabolic, exergonic, anabolic, endergonic reactions.
Adenosine Triphosphate (ATP) is the intermediate molecule that drives the exergonic transfer of energy to switch to endergonic anabolic reactions used in muscle contraction. This is what causes muscles to work which can require a breakdown, and also to build in the rest period, which occurs during the strengthening phase associated with muscular contraction. ATP is composed of adenine, a nitrogen containing base, ribose, a five carbon sugar (collectively called adenosine), and three phosphate groups. ATP is a high energy molecule because it stores large amounts of energy in the chemical bonds of the two terminal phosphate groups. The breaking of these chemical bonds in the Krebs Cycle provides the energy needed for muscular contraction.
[edit] Glucose
Because the ratio of hydrogen to oxygen atoms in all carbohydrates is always the same as that in water — that is, 2 to 1 — all of the oxygen consumed by the cells is used to oxidize the carbon in the carbohydrate molecule to form carbon dioxide. Consequently, during the complete oxidation of a glucose molecule, six molecules of carbon dioxide are produced and six molecules of oxygen are consumed.
The overall equation for this reaction is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Because the gas exchange in this reaction is equal, the respiratory quotient for carbohydrate is unity or 1.0:
- R.Q. = 6 CO2 / 6 O2
[edit] Fats
The chemical composition for fats differs from that of carbohydrates in that fats contain considerably fewer oxygen atoms in proportion to atoms of carbon and hydrogen. When listed on nutritional information tables, fats are generally divided into six categories: total fats, saturated fatty acid, polyunsaturated fatty acid, monounsaturated fatty acid, dietary cholesterol, and trans fatty acid. From a basal metabolic or resting metabolic perspective, more energy is needed to burn a saturated fatty acid than an unsaturated fatty acid. The fatty acid molecule is broken down and categorized based on the number of carbon atoms in its molecular structure. The chemical equation for metabolism of the twelve to sixteen carbon atoms in a saturated fatty acid molecule shows the difference between metabolism of carbohydrates and fatty acids. Palmitic acid is a commonly studied example of the saturated fatty acid molecule. When palmitic acid is broken down, more oxygen is needed and more carbon dioxide is produced, but the respiratory quotient moves below unity to account for the increased energy required to burn fat molecules (generally nine kilocalories per gram of fat versus four kilocalories for a gram of carbohydrate or protein.)
The overall equation for the substrate utilization of palmitic acid is:
- C16H32O2 + 23 O2 → 16 CO2 + 16 H2O
Thus the R.Q. for palmitic acid is 0.696:
- R.Q. = 16 CO2 / 23 O2 = 0.696
[edit] Proteins
Proteins are composed of carbon, hydrogen, oxygen, and nitrogen arranged in a variety of ways to form a large combination of amino acids. Unlike fat the body has no storage deposits of protein. All of it is contained in the body as important parts of tissues, blood hormones, and enzymes. The structural components of the body that contain these amino acids are continually undergoing a process of breakdown and replacement. The respiratory quotient for protein metabolism can be demonstrated by the chemical equation for oxidation of albumin:
C72H112N2O22S + 77 O2 → 63 CO2 + 38 H2O + SO3 + 9 CO(NH2)2
The R.Q. for albumin is 63 CO2/ 77 O2 = 0.818
The reason why this is important in the process of understanding protein metabolism is because the body can blend the three macronutrients and based on the mitochondrial density, a preferred ratio can be established which determines how much fuel is utilized in which packets for work accomplished by the muscles. Protein catabolism (breakdown) has been estimated to supply 10% to 15% of the total energy requirement during a two hour training session. However, if a person's muscle glycogen supplies are low from previous exercise sessions, the amount of energy derived from protein catabolism could increase from 15% to 45%. This process could severely degrade the protein structures needed to maintain survival such as contractile properties of proteins in the heart, cellular mitochondria, myoglobin storage, and metabolic enzymes within muscles.
The oxidative system (aerobic) is the primary source of ATP supplied to the body at rest and during low intensity activities and uses primarily carbohydrates and fats as substrates. Protein is not normally metabolized significantly, except during long term starvation and long bouts of exercise (greater than 90 minutes.) At rest approximately 70% of the ATP produced is derived from fats and 30% from carbohydrates. Following the onset of activity, as the intensity of the exercise increases, there is a shift in substrate preference from fats to carbohydrates. During high intensity aerobic exercise, almost 100% of the energy is derived from carbohydrates, if an adequate supply is available.
[edit] Exercise physiology
This section does not cite any references or sources. (October 2007)
Please improve this section by adding citations to reliable sources. Unverifiable material may be challenged and removed.
There are several companies testing the public for the respiratory quotient that identifies heart rates attributed to substrate utilization to assist with weight loss. It is theorized that if a person can more accurately know what amount of energy from carbohydrates, fats and proteins is needed to survive, then a person can select consumption patterns to more efficiently match what is required by the body for daily activities. Thus the emphasis shifts from caloric restriction, which slows the BMR or RMR and causes frustration of weight management goals, to substrate utilization, which focuses on what the body needs to stay healthy. By measuring the carbon dioxide expended (VCO2) in ml/min and dividing that by oxygen consumed (VO2) in ml/min you can determine the R.Q., which can then be compared to heart rate for purposes of application. The Balke VO2 Max running test could help to estimate what cardiac output level could be achieved by a 15 minute level of exertion using the following equation: (((Total distance covered ÷ 15) - 133) × 0.172) + 33.3. For a 50 year old male, weighing 150 pounds (68 kg), standing 69¾ inches (177 cm), that would be 47 ml/kg/min if he ran 3200 meters in 15 min. However, the same test using gas analysis would reveal more accurate information such as a peak VO2 of 51.8 ml/kg/min at an anaerobic threshold of 126 beats per minute, at 30.2 ml/kg/min and 58% of VO2 max. This would be 1725 meters in 15 minutes according to the Balke formula. But only gas analysis could determine the value accurately for purposes of losing weight successfully if that was an objective. So if a person had a measured BMR or RMR of 1610 kcal by gas analysis, and they walked around a track for 10 minutes with a heart rate at 94 beats per minute, they would consume all 25 grams of fat in a single quarter pounder with cheese with a previously determined anaerobic threshold of 126 beats per minute from a Peak VO2 of 51.8 ml/kg/minute. This analysis is precisely what is lacking from the current regime of dieting programs that stress caloric restriction, total calorie management from scale measure, and RMR or BMR from formulas using height, weight, age, activity level. These methods fail to appreciate the Krebs cycle and the ability of the body to adapt to lifestyle choices through BMR and RMR adjustment. By measuring the body with gas analysis as the principal determinant of BMR under strict fasting conditions, or RMR using less stringent measures, a person who wants to achieve a more optimal level of conditioning is more accurately directed to energy utilization patterns that are effective.
The reason why it's important to understand this difference with exercise testing is because it's essential to take into consideration whether or not the heart is capable of providing exercise stressed muscles with enough oxygen. Conditions such as obesity will affect the ability of formulas to accurately predict external work because the need to move a larger body changes the oxygen cost during exercise at least 5.8 ml/min for each kg of body weight.
[edit] Aerobic vs. anaerobic exercise
Studies published in 1992[3] and 1997[4] indicate that the level of aerobic fitness of an individual does not have any correlation with the level of resting metabolism. Both studies find that aerobic fitness levels do not improve the predictive power of Fat Free Mass for resting metabolic rate.
This suggests that anaerobic exercise may be more effective in raising the resting metabolic rate (Basal Metabolic Rate). Anaerobic exercise, such as weight lifting, builds additional muscle mass, which is Fat Free Mass. Additional Fat Free Mass will lead to a higher resting metabolic rate according to the above studies. Also, while aerobic exercise is beneficial for cardiovascular reasons as well as direct calorie burning, the above studies indicate it is not useful for increasing resting metabolism.
[edit] Longevity
In 1926 Raymond Pearl proposed that longevity varies inversely with basal metabolic rate (the "rate of living hypothesis"). Support for this hypothesis comes from the fact that mammals with larger body size have longer maximum life spans and the fact that the longevity of fruit flies varies inversely with ambient temperature.[5] Additionally, the life span of houseflies can be extended by preventing physical activity.[6]
But the ratio of resting metabolic rate to total daily energy expenditure can vary between 1.6 to 8.0 between species of mammals. Animals also vary in the degree of coupling between oxidative phosphorylation and ATP production, the amount of saturated fat in mitochondrial membranes, the amount of DNA repair, and many other factors that affect maximum life span.[7]
Organism Longevity and Basal Metabolic Rate
In allometric scaling maximum potential life span (MPLS) is directly related to metabolic rate (MR), where MR is the recharge rate of a biomass made up of covalent bonds subject to deterioration over time from thermodynamic, entropic pressure. Metabolism is essentially about redox coupling, and has nothing to do with thermogenesis. Metabolic efficiency (ME) is then expressed as the efficiency of this coupling, a ratio of amperes captured and used by biomass (W), to the amperes available for that purpose. MR is measured in watts, W is measured in grams. These factors are combined in a power law, an elaboration on Kleiber's Law relating MR to W and MPLS, that appears as MR = W^ (4ME-1)/4ME. When ME is 100%, MR = W^3/4, what is known popularly as quarter power scaling, a version of allometric scaling premised upon unreal estimations of biological efficiency.
The equation reveals that as ME drops below 20%, for W < one gram, MR/MPLS increases so dramatically as to endow W with virtual immortality by 16%. The smaller W is to begin with, the more dramatic is the increase in MR as ME diminishes. All of the cells of an organism fit into this range, i.e., less than one gram, and so this MR will be referred to as BMR.
But the equation reveals that as ME increases over 25%, BMR approaches zero. The equation also shows that for all W > one gram, where W is the organization of all of the BMRs of the organism's structure, but also includes the activity of the structure, as ME increases over 25%, MR/MPLS increases rather than decreases, as it does for BMR. An MR made up of an organization of BMRs will be referred to as an FMR. As ME decreases below 25%, FMR diminishes rather than increases as it does for BMR.
The antagonism between FMR and BMR is what marks the process of aging of biomass W in energetic terms. The ME for the organism is the same as that for the cells, such that the success of the organism's ability to find food (and lower its ME), is key to maintaining the BMR of the cells driven, otherwise, by starvation, to approaching zero; while at the same time a lower ME diminishes the FMR/MPLS of the organism.
[edit] Medical considerations
Each person's metabolism is unique due to their unique physical makeup and physical behavior. For some, this makes weight management a very difficult undertaking requiring sophisticated expertise. There are a number of medical adjustments to natural human processes that can affect one's metabolism.
Menopause affects metabolism but in different ways for different people, thus hormones are sometimes used to minimize the effects of menopause. Weight training can have a longer impact on metabolism than aerobic training, but there are no formulas currently written which can predict the length and duration of a raised metabolism from trophic changes with anabolic neuromuscular training. Gastric bypass surgery is used to reduce the content capacity of the stomach, bringing caloric intake down and lowering thermogenesis. Because the surgery significantly reduces caloric consumption, it will decrease BMR and RMR over time in the same fashion as aging, because the volume of the stomach is reduced. The stomach along with the rest of the digestive tract is a major contributor to BMR and RMR.
Celiac disease is fairly common, occurring in 1% of the U.S. population, with 2 million undiagnosed.[8][citation needed] The symptoms include unexplained weight loss, fatigue, and general lethargy. Sometimes symptoms are accompanied by a ravenous appetite or abdominal cramping, bloating, and gas because of continued decomposition of food and partially digested bowel contents. Celiac disease is caused by an autoimmune response to certain proteins found in grains, including wheat, rye, and barley. The cells in the small intestine are most affected. The small intestine is important in absorbing food nutrients and various body fluids. Healthy small intestines are lined with small projections (villi) that increase surface area and absorption. Damage to these projections causes malnutrition, diarrhea, and dehydration. Totally eliminating gluten proteins from the diet will prevent irritation to the villi and cause the symptoms to cease. Celiac disease along with other disease processes lower and reduce BMR and RMR.
[edit] Cardiovascular implications
Heart rate is determined by the medulla oblongata and part of the pons, two organs located inferior to the hypothalamus on the brain stem. Heart rate is important for basal metabolic rate and resting metabolic rate because it drives the blood supply, stimulating the Krebs cycle. During exercise that achieves the anaerobic threshold, it is possible to deliver substrates that are desired for optimal energy utilization. The anaerobic threshold is defined as the energy utilization level of heart rate exertion that occurs without oxygen during a standardized test with a specific protocol for accuracy of measurement, such as the Bruce Treadmill protocol (see Metabolic equivalent). With four to six weeks of targeted training the body systems can adapt to a higher perfusion of mitochondrial density for increased oxygen availability for the Krebs cycle, or tricarboxylic cycle, or the glycolitic cycle. This in turn leads to a lower resting heart rate, lower blood pressure, and increased resting or basal metabolic rate.
Knowing what the body burns at rest or through exercise yields (via heart rate monitoring) a targeted program of energy utilization based on metabolic performance. The resting heart rate is correlated to the resting metabolic rate because of the singular contribution made by the heart to survival. By measuring heart rate we can then derive estimations of what level of substrate utilization is actually causing biochemical metabolism in our bodies at rest or in activity. This in turn can help a person to maintain an appropriate level of consumption and utilization by studying a graphical representation of the anaerobic threshold. This can be confirmed by blood tests and gas analysis using either direct or indirect calorimetry to show the effect of substrate utilization. The measures of basal metabolic rate and resting metabolic rate are becoming essential tools for maintaining a healthy body weight.
Basal metabolic rate (BMR) is the amount of energy expended while at rest in a neutrally temperate environment, in the post-absorptive state (meaning that the digestive system is inactive, which requires about twelve hours of fasting in humans). The release of energy in this state is sufficient only for the functioning of the vital organs, such as the heart, lungs, brain and the rest of the nervous system, liver, kidneys, sex organs, muscles and skin. BMR decreases with age and with the loss of lean body mass. Increased cardiovascular exercise and muscle mass can increase BMR. Illness, previously consumed food and beverages, environmental temperature, and stress levels can affect one's overall energy expenditure, and can affect one's BMR.
BMR is measured under very restrictive circumstances when a person is awake, but at complete rest. An accurate BMR measurement requires that the person's sympathetic nervous system is not stimulated. A more common and closely related measurement, used under less strict conditions, is resting metabolic rate (RMR).[1]
BMR and RMR are measured by gas analysis through either direct or indirect calorimetry, though a rough estimation can be acquired through an equation using age, sex, height, and weight. Studies of energy metabolism using both methods provide convincing evidence for the validity of the respiratory quotient (R.Q.), which measures the inherent composition and utilization of carbohydrates, fats and proteins as they are converted to energy substrate units that can be used by the body as energy.
Contents[hide] |
[edit] Nutrition and dietary considerations
Basal metabolic rate is usually by far the largest component of total caloric expenditure. However, the Harris-Benedict equations are only approximate and variation in BMR (reflecting varying body composition), in physical activity levels, and in energy expended in thermogenesis make it difficult to estimate the dietary consumption any particular individual needs in order to maintain body weight. 2000 kilocalories is often quoted but is no more than a guideline.
[edit] Physiology
Both basal metabolic rate and resting metabolic rate are usually expressed in terms of daily rates of energy expenditure. The early work of the scientists J. Arthur Harris and Francis G. Benedict showed that approximate values could be derived using body surface area (computed from height and weight), age, and sex, along with the oxygen and carbon dioxide measures taken from calorimetry. Studies also showed that by eliminating the sex differences that occur with the accumulation of adipose tissue by expressing metabolic rate per unit of "fat-free" or lean body weight, the values between sexes for basal metabolism are essentially the same. Exercise physiology textbooks have tables to show the conversion of height and body surface area as they relate to weight and basal metabolic values.
The primary organ responsible for regulating metabolism is the hypothalamus. The hypothalamus is located on the brain stem and forms the floor and part of the lateral walls of the third ventricle of the cerebrum. The chief functions of the hypothalamus are:
-
- control and integration of activities of the autonomic nervous system (ANS)
- The ANS regulates contraction of smooth muscle and cardiac muscle, along with secretions of many endocrine organs such as the thyroid gland (associated with many metabolic disorders).
- Through the ANS, the hypothalamus is the main regulator of visceral activities, such as heart rate, movement of food through the gastrointestinal tract, and contraction of the urinary bladder.
- production and regulation of feelings of rage and aggression
- regulation of body temperature
- regulation of food intake, through two centers:
- The feeding center or hunger center is responsible for the sensations that cause us to seek food. When sufficient food or substrates have been received and leptin is high, then the satiety center is stimulated and sends impulses that inhibit the feeding center. When insufficient food is present in the stomach and ghrelin levels are high, receptors in the hypothalamus initiate the sense of hunger.
- The thirst center operates similarly when certain cells in the hypothalamus are stimulated by the rising osmotic pressure of the extracellular fluid. If thirst is satisfied, osmotic pressure decreases.
- control and integration of activities of the autonomic nervous system (ANS)
All of these functions taken together form a survival mechanism that causes us to sustain the body processes that BMR and RMR measure.
[edit] BMR estimation formulas
Several prediction equations exist. Historically most notable was Harris-Benedict equation, which was created in 1919.
The original equations from Harris and Benedict are:
-
- for men,

- for women,
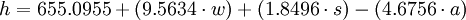
- for men,
where h = total heat production per 24 hours at complete rest in kilocalories (kcal), w = weight in kilograms, s = stature (height) in centimeters, and a = age in years, and with the difference in BMR for men and women being mainly due to differences in body weight.[2]
It was the best prediction equation until recently, when MD Mifflin and ST St Jeor in 1990 created new equation:
During last 100 years lifestyle changed and a survey in 2005 showed it to be about 5% more accurate.
[edit] Example calculation
For example, a 55 year old woman weighing 130 lb (59 kg) and 5 feet 6 inches (168 cm) tall would have a BMR of 1266 kcal per day or 52.8 kcal/h (61.3 watts). This is computed by as 655 + (9.6 x 59) + (1.8 x 168) - (4.7 x 55).
To calculate Daily Calorie Needs, this BMR value is multiplied by a factor with a value between 1.2 and 1.9, depending on the person's activity level.
An online BMR calculator for both metric and non-metric values can be found at this link [1]
[edit] Animal BMR
Kleiber's law relates the BMR for animals of different sizes and the observations indicate that the BMR is proportional to the 3/4 power of body mass. Warm blooded, cold blooded and unicellular animals fit on different curves.
[edit] Biochemistry
| Energy expenditure breakdown | |
|---|---|
| liver | 27% |
| brain | 19% |
| heart | 7% |
| kidneys | 10% |
| skeletal muscle | 18% |
| other organs | 19% |
About 70% of a human's total energy expenditure is due to the basal life processes within the organs of the body (see table). About 20% of one's energy expenditure comes from physical activity and another 10% from thermogenesis, or digestion of food.[citation needed] All of these processes require an intake of oxygen along with coenzymes to provide energy for survival (usually from macronutrients like carbohydrates, fats, and proteins) and expel carbon dioxide, which is explained by the Krebs cycle.
What enables the Krebs cycle to perform metabolic changes to fats, carbohydrates, and proteins is energy which can be defined as the ability or capacity to do work. The breakdown of large molecules into smaller molecules associated with release of energy is catabolism. The building up process is termed anabolism. The breakdown of proteins into amino acids is an example of catabolism while the formation of proteins from amino acids is an anabolic process.
Exergonic reactions are energy-releasing reactions and are generally catabolic. Endergonic reactions require energy and include anabolic reactions and the contraction of muscle. Metabolism is the total of all catabolic, exergonic, anabolic, endergonic reactions.
Adenosine Triphosphate (ATP) is the intermediate molecule that drives the exergonic transfer of energy to switch to endergonic anabolic reactions used in muscle contraction. This is what causes muscles to work which can require a breakdown, and also to build in the rest period, which occurs during the strengthening phase associated with muscular contraction. ATP is composed of adenine, a nitrogen containing base, ribose, a five carbon sugar (collectively called adenosine), and three phosphate groups. ATP is a high energy molecule because it stores large amounts of energy in the chemical bonds of the two terminal phosphate groups. The breaking of these chemical bonds in the Krebs Cycle provides the energy needed for muscular contraction.
[edit] Glucose
Because the ratio of hydrogen to oxygen atoms in all carbohydrates is always the same as that in water — that is, 2 to 1 — all of the oxygen consumed by the cells is used to oxidize the carbon in the carbohydrate molecule to form carbon dioxide. Consequently, during the complete oxidation of a glucose molecule, six molecules of carbon dioxide are produced and six molecules of oxygen are consumed.
The overall equation for this reaction is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Because the gas exchange in this reaction is equal, the respiratory quotient for carbohydrate is unity or 1.0:
- R.Q. = 6 CO2 / 6 O2
[edit] Fats
The chemical composition for fats differs from that of carbohydrates in that fats contain considerably fewer oxygen atoms in proportion to atoms of carbon and hydrogen. When listed on nutritional information tables, fats are generally divided into six categories: total fats, saturated fatty acid, polyunsaturated fatty acid, monounsaturated fatty acid, dietary cholesterol, and trans fatty acid. From a basal metabolic or resting metabolic perspective, more energy is needed to burn a saturated fatty acid than an unsaturated fatty acid. The fatty acid molecule is broken down and categorized based on the number of carbon atoms in its molecular structure. The chemical equation for metabolism of the twelve to sixteen carbon atoms in a saturated fatty acid molecule shows the difference between metabolism of carbohydrates and fatty acids. Palmitic acid is a commonly studied example of the saturated fatty acid molecule. When palmitic acid is broken down, more oxygen is needed and more carbon dioxide is produced, but the respiratory quotient moves below unity to account for the increased energy required to burn fat molecules (generally nine kilocalories per gram of fat versus four kilocalories for a gram of carbohydrate or protein.)
The overall equation for the substrate utilization of palmitic acid is:
- C16H32O2 + 23 O2 → 16 CO2 + 16 H2O
Thus the R.Q. for palmitic acid is 0.696:
- R.Q. = 16 CO2 / 23 O2 = 0.696
[edit] Proteins
Proteins are composed of carbon, hydrogen, oxygen, and nitrogen arranged in a variety of ways to form a large combination of amino acids. Unlike fat the body has no storage deposits of protein. All of it is contained in the body as important parts of tissues, blood hormones, and enzymes. The structural components of the body that contain these amino acids are continually undergoing a process of breakdown and replacement. The respiratory quotient for protein metabolism can be demonstrated by the chemical equation for oxidation of albumin:
C72H112N2O22S + 77 O2 → 63 CO2 + 38 H2O + SO3 + 9 CO(NH2)2
The R.Q. for albumin is 63 CO2/ 77 O2 = 0.818
The reason why this is important in the process of understanding protein metabolism is because the body can blend the three macronutrients and based on the mitochondrial density, a preferred ratio can be established which determines how much fuel is utilized in which packets for work accomplished by the muscles. Protein catabolism (breakdown) has been estimated to supply 10% to 15% of the total energy requirement during a two hour training session. However, if a person's muscle glycogen supplies are low from previous exercise sessions, the amount of energy derived from protein catabolism could increase from 15% to 45%. This process could severely degrade the protein structures needed to maintain survival such as contractile properties of proteins in the heart, cellular mitochondria, myoglobin storage, and metabolic enzymes within muscles.
The oxidative system (aerobic) is the primary source of ATP supplied to the body at rest and during low intensity activities and uses primarily carbohydrates and fats as substrates. Protein is not normally metabolized significantly, except during long term starvation and long bouts of exercise (greater than 90 minutes.) At rest approximately 70% of the ATP produced is derived from fats and 30% from carbohydrates. Following the onset of activity, as the intensity of the exercise increases, there is a shift in substrate preference from fats to carbohydrates. During high intensity aerobic exercise, almost 100% of the energy is derived from carbohydrates, if an adequate supply is available.
[edit] Exercise physiology
| This section does not cite any references or sources. (October 2007) Please improve this section by adding citations to reliable sources. Unverifiable material may be challenged and removed. |
There are several companies testing the public for the respiratory quotient that identifies heart rates attributed to substrate utilization to assist with weight loss. It is theorized that if a person can more accurately know what amount of energy from carbohydrates, fats and proteins is needed to survive, then a person can select consumption patterns to more efficiently match what is required by the body for daily activities. Thus the emphasis shifts from caloric restriction, which slows the BMR or RMR and causes frustration of weight management goals, to substrate utilization, which focuses on what the body needs to stay healthy. By measuring the carbon dioxide expended (VCO2) in ml/min and dividing that by oxygen consumed (VO2) in ml/min you can determine the R.Q., which can then be compared to heart rate for purposes of application. The Balke VO2 Max running test could help to estimate what cardiac output level could be achieved by a 15 minute level of exertion using the following equation: (((Total distance covered ÷ 15) - 133) × 0.172) + 33.3. For a 50 year old male, weighing 150 pounds (68 kg), standing 69¾ inches (177 cm), that would be 47 ml/kg/min if he ran 3200 meters in 15 min. However, the same test using gas analysis would reveal more accurate information such as a peak VO2 of 51.8 ml/kg/min at an anaerobic threshold of 126 beats per minute, at 30.2 ml/kg/min and 58% of VO2 max. This would be 1725 meters in 15 minutes according to the Balke formula. But only gas analysis could determine the value accurately for purposes of losing weight successfully if that was an objective. So if a person had a measured BMR or RMR of 1610 kcal by gas analysis, and they walked around a track for 10 minutes with a heart rate at 94 beats per minute, they would consume all 25 grams of fat in a single quarter pounder with cheese with a previously determined anaerobic threshold of 126 beats per minute from a Peak VO2 of 51.8 ml/kg/minute. This analysis is precisely what is lacking from the current regime of dieting programs that stress caloric restriction, total calorie management from scale measure, and RMR or BMR from formulas using height, weight, age, activity level. These methods fail to appreciate the Krebs cycle and the ability of the body to adapt to lifestyle choices through BMR and RMR adjustment. By measuring the body with gas analysis as the principal determinant of BMR under strict fasting conditions, or RMR using less stringent measures, a person who wants to achieve a more optimal level of conditioning is more accurately directed to energy utilization patterns that are effective.
The reason why it's important to understand this difference with exercise testing is because it's essential to take into consideration whether or not the heart is capable of providing exercise stressed muscles with enough oxygen. Conditions such as obesity will affect the ability of formulas to accurately predict external work because the need to move a larger body changes the oxygen cost during exercise at least 5.8 ml/min for each kg of body weight.
[edit] Aerobic vs. anaerobic exercise
Studies published in 1992[3] and 1997[4] indicate that the level of aerobic fitness of an individual does not have any correlation with the level of resting metabolism. Both studies find that aerobic fitness levels do not improve the predictive power of Fat Free Mass for resting metabolic rate.
This suggests that anaerobic exercise may be more effective in raising the resting metabolic rate (Basal Metabolic Rate). Anaerobic exercise, such as weight lifting, builds additional muscle mass, which is Fat Free Mass. Additional Fat Free Mass will lead to a higher resting metabolic rate according to the above studies. Also, while aerobic exercise is beneficial for cardiovascular reasons as well as direct calorie burning, the above studies indicate it is not useful for increasing resting metabolism.
[edit] Longevity
In 1926 Raymond Pearl proposed that longevity varies inversely with basal metabolic rate (the "rate of living hypothesis"). Support for this hypothesis comes from the fact that mammals with larger body size have longer maximum life spans and the fact that the longevity of fruit flies varies inversely with ambient temperature.[5] Additionally, the life span of houseflies can be extended by preventing physical activity.[6]
But the ratio of resting metabolic rate to total daily energy expenditure can vary between 1.6 to 8.0 between species of mammals. Animals also vary in the degree of coupling between oxidative phosphorylation and ATP production, the amount of saturated fat in mitochondrial membranes, the amount of DNA repair, and many other factors that affect maximum life span.[7]
Organism Longevity and Basal Metabolic Rate
In allometric scaling maximum potential life span (MPLS) is directly related to metabolic rate (MR), where MR is the recharge rate of a biomass made up of covalent bonds subject to deterioration over time from thermodynamic, entropic pressure. Metabolism is essentially about redox coupling, and has nothing to do with thermogenesis. Metabolic efficiency (ME) is then expressed as the efficiency of this coupling, a ratio of amperes captured and used by biomass (W), to the amperes available for that purpose. MR is measured in watts, W is measured in grams. These factors are combined in a power law, an elaboration on Kleiber's Law relating MR to W and MPLS, that appears as MR = W^ (4ME-1)/4ME. When ME is 100%, MR = W^3/4, what is known popularly as quarter power scaling, a version of allometric scaling premised upon unreal estimations of biological efficiency.
The equation reveals that as ME drops below 20%, for W < one gram, MR/MPLS increases so dramatically as to endow W with virtual immortality by 16%. The smaller W is to begin with, the more dramatic is the increase in MR as ME diminishes. All of the cells of an organism fit into this range, i.e., less than one gram, and so this MR will be referred to as BMR.
But the equation reveals that as ME increases over 25%, BMR approaches zero. The equation also shows that for all W > one gram, where W is the organization of all of the BMRs of the organism's structure, but also includes the activity of the structure, as ME increases over 25%, MR/MPLS increases rather than decreases, as it does for BMR. An MR made up of an organization of BMRs will be referred to as an FMR. As ME decreases below 25%, FMR diminishes rather than increases as it does for BMR.
The antagonism between FMR and BMR is what marks the process of aging of biomass W in energetic terms. The ME for the organism is the same as that for the cells, such that the success of the organism's ability to find food (and lower its ME), is key to maintaining the BMR of the cells driven, otherwise, by starvation, to approaching zero; while at the same time a lower ME diminishes the FMR/MPLS of the organism.
[edit] Medical considerations
Each person's metabolism is unique due to their unique physical makeup and physical behavior. For some, this makes weight management a very difficult undertaking requiring sophisticated expertise. There are a number of medical adjustments to natural human processes that can affect one's metabolism.
Menopause affects metabolism but in different ways for different people, thus hormones are sometimes used to minimize the effects of menopause. Weight training can have a longer impact on metabolism than aerobic training, but there are no formulas currently written which can predict the length and duration of a raised metabolism from trophic changes with anabolic neuromuscular training. Gastric bypass surgery is used to reduce the content capacity of the stomach, bringing caloric intake down and lowering thermogenesis. Because the surgery significantly reduces caloric consumption, it will decrease BMR and RMR over time in the same fashion as aging, because the volume of the stomach is reduced. The stomach along with the rest of the digestive tract is a major contributor to BMR and RMR.
Celiac disease is fairly common, occurring in 1% of the U.S. population, with 2 million undiagnosed.[8][citation needed] The symptoms include unexplained weight loss, fatigue, and general lethargy. Sometimes symptoms are accompanied by a ravenous appetite or abdominal cramping, bloating, and gas because of continued decomposition of food and partially digested bowel contents. Celiac disease is caused by an autoimmune response to certain proteins found in grains, including wheat, rye, and barley. The cells in the small intestine are most affected. The small intestine is important in absorbing food nutrients and various body fluids. Healthy small intestines are lined with small projections (villi) that increase surface area and absorption. Damage to these projections causes malnutrition, diarrhea, and dehydration. Totally eliminating gluten proteins from the diet will prevent irritation to the villi and cause the symptoms to cease. Celiac disease along with other disease processes lower and reduce BMR and RMR.
[edit] Cardiovascular implications
Heart rate is determined by the medulla oblongata and part of the pons, two organs located inferior to the hypothalamus on the brain stem. Heart rate is important for basal metabolic rate and resting metabolic rate because it drives the blood supply, stimulating the Krebs cycle. During exercise that achieves the anaerobic threshold, it is possible to deliver substrates that are desired for optimal energy utilization. The anaerobic threshold is defined as the energy utilization level of heart rate exertion that occurs without oxygen during a standardized test with a specific protocol for accuracy of measurement, such as the Bruce Treadmill protocol (see Metabolic equivalent). With four to six weeks of targeted training the body systems can adapt to a higher perfusion of mitochondrial density for increased oxygen availability for the Krebs cycle, or tricarboxylic cycle, or the glycolitic cycle. This in turn leads to a lower resting heart rate, lower blood pressure, and increased resting or basal metabolic rate.
Knowing what the body burns at rest or through exercise yields (via heart rate monitoring) a targeted program of energy utilization based on metabolic performance. The resting heart rate is correlated to the resting metabolic rate because of the singular contribution made by the heart to survival. By measuring heart rate we can then derive estimations of what level of substrate utilization is actually causing biochemical metabolism in our bodies at rest or in activity. This in turn can help a person to maintain an appropriate level of consumption and utilization by studying a graphical representation of the anaerobic threshold. This can be confirmed by blood tests and gas analysis using either direct or indirect calorimetry to show the effect of substrate utilization. The measures of basal metabolic rate and resting metabolic rate are becoming essential tools for maintaining a healthy body weight.
'연구하는 인생 > 西醫學 Medicine' 카테고리의 다른 글
| 무릎 관절염 고칠 수 있을까 (0) | 2008.03.31 |
|---|---|
| 퇴행성 골관절염 -무릎의 퇴행성 관절염 (0) | 2008.03.31 |
| Achilles Tendon: Tendinitis and Tears (0) | 2008.03.26 |
| Posterior Tibial Tendon Problems (0) | 2008.03.26 |
| Achilles Tendonitis and Achilles Tendon Rupture (0) | 2008.03.26 |


