Insulin therapy
From Wikipedia, the free encyclopedia
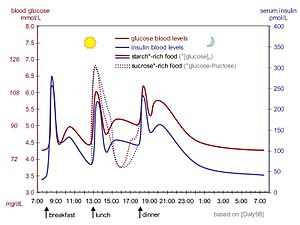
Insulin therapy, refers to treatment of diabetes by administration of exogenous insulin.
Insulin is used medically to treat some forms of diabetes mellitus. Patients with Type 1 diabetes mellitus depend on external insulin (most commonly injected subcutaneously) for their survival because the hormone is no longer produced internally. Patients with Type 2 diabetes mellitus are insulin resistant, have relatively low insulin production, or both; some patients with Type 2 diabetes may eventually require insulin when other medications fail to control blood glucose levels adequately.
Contents[hide] |
[edit] Principles
Insulin is required for all animal life (excluding certain insects). Its mechanism of action is almost identical in nematode worms (e.g.C. elegans), fish, and mammals, and it is a protein that has been highly conserved across evolutionary time. Insulin must be administered to patients who experience such a deprivation. Clinically, this condition is called diabetes mellitus type 1.
The initial sources of insulin for clinical use in humans were cow, horse, pig or fish pancreases. Insulin from these sources is effective in humans as it is nearly identical to human insulin (three amino acid difference in bovine insulin, one amino acid difference in porcine). Differences in suitability of beef-, pork-, or fish-derived insulin for individual patients have historically been due to lower preparation purity resulting in allergic reactions to the presence of non-insulin substances. Though purity has improved steadily since the 1920s ultimately reaching purity of 99% by the mid-1970s thanks to high-pressure liquid chromatography (HPLC) methods, but minor allergic reactions still occur occasionally, although the same types of allergic reactions have also been known to occur in response to synthetic "human" insulin varieties. Insulin production from animal pancreases was widespread for decades, but very few patients today rely on insulin from animal sources, largely because few pharmaceutical companies sell it anymore.
Synthetic "human" insulin is now manufactured for widespread clinical use using genetic engineering techniques using recombinant DNA technology, which the manufacturers claim reduces the presence of many impurities. Eli Lilly marketed the first such insulin, Humulin, in 1982. Humulin was the first medication produced using modern genetic engineering techniques in which actual human DNA is inserted into a host cell (E. coli in this case). The host cells are then allowed to grow and reproduce normally, and due to the inserted human DNA, they produce a synthetic version of human insulin. However, the clinical preparations prepared from such insulins differ from endogenous human insulin in several important respects; an example is the absence of C-peptide which has in recent years been shown to have systemic effects itself.
Genentech developed the technique Lilly used to produce Humulin, although the company never commercially marketed the product themselves.Novo Nordisk has also developed a genetically engineered insulin independently. According to a survey that the International Diabetes Federation conducted in 2002 on the access to and availability of insulin in its member countries, approximately 70% of the insulin that is currently sold in the world is recombinant, biosynthetic 'human' insulin.[1] A majority of insulin used clinically today is produced this way, although the clinical evidence has provided conflicting evidence on whether these insulins are any less likely to produce an allergic reaction. Also, the International Diabetes Federation's position statement is very clear in stating that "there is NO overwhelming evidence to prefer one species of insulin over another" and "[modern, highly-purified] animal insulins remain a perfectly acceptable alternative."[2]
Since January 2006, all insulins distributed in the U.S. and some other countries are synthetic "human" insulins or their analogs. A specialFDA importation process is required to obtain bovine or porcine derived insulin for use in the U.S., although there may be some remaining stocks of porcine insulin made by Lilly in 2005 or earlier.
There are several problems with insulin as a clinical treatment for diabetes:
- Mode of administration.
- Selecting the 'right' dose and timing.
- Selecting an appropriate insulin preparation (typically on 'speed of onset and duration of action' grounds).
- Adjusting dosage and timing to fit food intake timing, amounts, and types.
- Adjusting dosage and timing to fit exercise undertaken.
- Adjusting dosage, type, and timing to fit other conditions, for instance the increased stress of illness.
- Variability in absorption into the bloodstream via subcutaneous delivery
- The dosage is non-physiological in that a subcutaneous bolus dose of insulin alone is administered instead of combination of insulin and C-peptide being released gradually and directly into the portal vein.
- It is simply a nuisance for patients to inject whenever they eat carbohydrate or have a high blood glucose reading.
- It is dangerous in case of mistake (most especially 'too much' insulin).
[edit] Types
Medical preparations of insulin (from the major suppliers – Eli Lilly, Novo Nordisk, and Sanofi Aventis – or from any other) are never just 'insulin in water'. Clinical insulins are specially prepared mixtures of insulin plus other substances including preservatives. These delay absorption of the insulin, adjust the pH of the solution to reduce reactions at the injection site, and so on.
Slight variations of the human insulin molecule are called insulin analogs, (technically "insulin receptor ligands") so named because they are not technically insulin, rather they are analogs which retain the hormone's glucose management functionality. They have absorption and activity characteristics not currently possible with subcutaneously injected insulin proper. They are either absorbed rapidly in an attempt to mimic real beta cell insulin (as with Lilly's lispro, Novo Nordisk's aspart and Sanofi Aventis' glulisine), or steadily absorbed after injection instead of having a 'peak' followed by a more or less rapid decline in insulin action (as with Novo Nordisk's version Insulin detemir and Sanofi Aventis's Insulin glargine), all while retaining insulin's glucose-lowering action in the human body. However, a number of meta-analyses, including those done by the Cochrane Collaboration in the United Kingdom in 2002,[3] Germany's Institute for Quality and Cost Effectiveness in the Health Care Sector [IQWiG] released in 2007,[4] and the Canadian Agency for Drugs and Technology in Health (CADTH)[5]also released in 2007 have shown no unequivocal advantages in clinical use of insulin analogs over more conventional insulin types.
Choosing insulin type and dosage/timing should be done by an experienced medical professional working closely with the diabetic patient.
The commonly used types of insulin are:
- Rapid-acting types are presently insulin analogs, such as the insulin analogs aspart or lispro. these begin to work within 5 to 15 minutes and are active for 3 to 4 hours. Most insulins form "clumps" which delay entry into the blood in active form; these analog insulins do not, but have normal insulin activity. Newer varieties are in now in Phase II clinical trials which are designed to work rapidly, but retain the same genetic structure as regular human insulin.[6]
- Short-acting, such as regular insulin – starts working within 30 minutes and is active about 5 to 8 hours.
- Intermediate-acting, such as NPH, or semilente insulin – starts working in 1 to 3 hours and is active 16 to 24 hours.
- Long-acting, such as ultralente insulin – starts working in 4 to 6 hours, and is active well beyond 32 hours.
- Insulin glargine and Insulin detemir – both insulin analogs which start working within 1 to 2 hours and continue to be active, without major peaks or dips, for about 24 hours, although this varies in many individuals.
- A mixture of NPH and regular insulin – starts working in 30 minutes and is active 16 to 24 hours. There are several variations with different proportions of the mixed insulins.
- A mixture of Semilente and Ultralente (typically in the proportion 30% Semilente to 70% Ultralente), known as Lente, is typically active for an entire 24 hour period. Beef Lente, in particular, has a very 'flat' profile.[citation needed]
[edit] Yeast-based
In late 2003, Wockhardt commenced manufacture of a yeast-based insulin costing $3.25 in India claiming it eliminates the risk of contracting diseases such as BSE and CJD associated with insulin derived from pigs and cattle.[7] However, the company continues to manufacture insulin derived from pigs in the United Kingdom.
[edit] Modes of administration
Unlike many medicines, insulin cannot be taken orally. Like nearly all other proteins introduced into the gastrointestinal tract, it is reduced to fragments (even single amino acid components), whereupon all 'insulin activity' is lost. There has been some research into ways to protect insulin from the digestive tract, so that it can be administered in a pill. So far this is entirely experimental.
[edit] Subcutaneous
Insulin is usually taken as subcutaneous injections by single-use syringes with needles, an insulin pump, or by repeated-use insulin pens with needles. Patients who wish to reduce repeated skin puncture of insulin injections often use an injection port in conjunction with syringes.
Administration schedules often attempt to mimic the physiologic secretion of insulin by the pancreas. Hence, both a long-acting insulin and a short-acting insulin are typically used.
[edit] Insulin pump
Insulin pumps are a reasonable solution for some. Advantages to the patient are better control over background or 'basal' insulin dosage, bolus doses calculated to fractions of a unit, and calculators in the pump that may help with determining 'bolus' infusion dosages. The limitations are cost, the potential for hypoglycemic and hyperglycemic episodes, catheter problems, and no "closed loop" means of controlling insulin delivery based on current blood glucose levels.
Insulin pumps may be like 'electrical injectors' attached to a temporarily implanted catheter or cannula. Some who cannot achieve adequate glucose control by conventional (or jet) injection are able to do so with the appropriate pump.
As with injections, if too much insulin is delivered or the patient eats less than he or she dosed for, there will be hypoglycemia. on the other hand, if too little insulin is delivered, there will be hyperglycemia. Both can be life-threatening. In addition, indwelling catheters pose the risk of infection and ulceration, and some patients may also develop lipodystrophy due to the infusion sets. These risks can often be minimized by keeping infusion sites clean. Insulin pumps require care and effort to use correctly. However, some patients with diabetes are capable of keeping their glucose in reasonable control only with an insulin pump.
[edit] Inhalation
In 2006 the U.S. Food and Drug Administration approved the use of Exubera, the first inhalable insulin.[8] It has been withdrawn from the market by its maker as of 3Q 2007, due to lack of acceptance.
Inhaled insulin has similar efficacy to injected insulin, both in terms of controlling glucose levels and blood half-life. Currently, inhaled insulin is short acting and is typically taken before meals; an injection of long-acting insulin at night is often still required.[9] When patients were switched from injected to inhaled insulin, no significant difference was found in HbA1c levels over three months. Accurate dosing is still a problem, although patients showed no significant weight gain or pulmonary function decline over the length of the trial, when compared to the baseline.[10] Following its commercial launch in 2005 in the UK, it was not (as of July 2006) recommended by National Institute for Health and Clinical Excellence for routine use, except in cases where there is "proven injection phobia diagnosed by a psychiatrist or psychologist".[9]
In January 2008, the world's largest insulin manufacturer, Novo Nordisk A/S, also announced that the company was discontinuing all further development of the company's own version of inhalable insulin, known as the AERx iDMS inhaled insulin system.[11] Similarly, Eli Lilly and Company ended its efforts to develop its Air inhaled insulin in March 2008.[12] MannKind Corp. (whose majority owner, Alfred E. Mann, remained optimistic about the concept.[13]
[edit] Transdermal
There are several methods for transdermal delivery of insulin. Pulsatile insulin uses microjets to pulse insulin into the patient, mimicking the physiological secretions of insulin by the pancreas.[14] Jet injection had different insulin delivery peaks and durations as compared to needle injection. Some diabetics find control possible with jet injectors, but not with hypodermic injection.
Both electricity using iontophoresis[15] and ultrasound have been found to make the skin temporarily porous. The insulin administration aspect remains experimental, but the blood glucose test aspect of 'wrist appliances' is commercially available.
Researchers have produced a watch-like device that tests for blood glucose levels through the skin and administers corrective doses of insulin through pores in the skin.
[edit] Intranasal insulin
Intranasal insulin is being investigated.[16]
[edit] Oral insulin
The basic appeal of oral hypoglycemic agents is that most people would prefer a pill to an injection. However, insulin is a protein, which are digested in the stomach and gut and in order to be effective at controlling blood sugar, can not be taken orally.
The potential market for an oral form of insulin is assumed to be enormous, thus many laboratories have attempted to devise ways of moving enough intact insulin from the gut to the portal vein to have a measurable effect on blood sugar. As of 2004, no products appear to be successful enough yet to bring to market.[17]
A Connecticut-based biopharmaceutical company called Biodel, Inc. is developing what it calls VIAtab, an oral formulation of insulin designed to be administered sublingually. This therapy is a tablet that dissolves in minutes when placed under the tongue. In a Phase I study, VIAtab delivered insulin to the blood stream quickly and resembled the first-phase insulin release spike found in healthy individuals. The company claims that an oral insulin therapy would be more convenient than currently available injectable or inhalable therapies, and they expect that convenience to result in increased insulin usage among the currently underserved early-stage patients with Type 2 diabetes, thus helping to create better long-term outcomes for that patient population.[18]
An Israeli pharmaceutical company, Oramed Pharmaceuticals, is currently conducting Phase 2A studies on an oral insulin pill[19]. Initial trials indicate that the new chemical make-up of the coating surrounding the pill prevent breakdown until the medicine reaches the intestines. once reaching the intestine, the capsule releases insulin which is absorbed through the portal vein and into the liver.
Australian biopharmaceutical company, Apollo Life Sciences, plans to enter the Phase I trial of its oral insulin tablet in mid-2008.[20]
Biocon, Asia's largest Biopharmaceutical company, based out of Bangalore, India is also developing an Oral Insulin Product. It has just completed Phase IIa trials.
[edit] Pancreatic transplantation
Another improvement would be a transplantation of the pancreas or beta cell to avoid periodic insulin administration. This would result in a self-regulating insulin source. Transplantation of an entire pancreas (as an individual organ) is difficult and relatively uncommon. It is often performed in conjunction with liver or kidney transplant, although it can be done by itself. It is also possible to do a transplantation of only the pancreatic beta cells. However, islet transplants had been highly experimental (read 'prone to failure') for many years, but some researchers in Alberta, Canada, have developed techniques with a high initial success rate (about 90% in one group). Nearly half of those who got an islet cell transplant were insulin-free one year after the operation; by the end of the second year that number drops to about one in seven. However, researchers at the University of Illinois at Chicago (UIC) have slightly modified the Edmonton Protocol procedure for islet cell transplantation and achieved insulin independence in diabetes patients with fewer but better-functioning pancreatic islet cells[21]. Longer-term studies are needed to validate whether it improves the rate of insulin-independence.
Beta cell transplant may become practical in the near future. Additionally, some researchers have explored the possibility of transplantinggenetically engineered non-beta cells to secrete insulin.[22] Clinically testable results are far from realization at this time. Several other non-transplant methods of automatic insulin delivery are being developed in research labs, but none is close to clinical approval.
[edit] Artificial pancreas
[edit] Dosage and timing
The central problem for those requiring external insulin is picking the right dose of insulin and the right timing.
Physiological regulation of blood glucose, as in the non-diabetic, would be best. Increased blood glucose levels after a meal is a stimulus for prompt release of insulin from the pancreas. The increased insulin level causes glucose absorption and storage in cells, reduces glycogen to glucose conversion, reducing blood glucose levels, and so reducing insulin release. The result is that the blood glucose level rises somewhat after eating, and within an hour or so, returns to the normal 'fasting' level. Even the best diabetic treatment with synthetic human insulin or even insulin analogs, however administered, falls far short of normal glucose control in the non-diabetic.
Complicating matters is that the composition of the food eaten (see glycemic index) affects intestinal absorption rates. Glucose from some foods is absorbed more (or less) rapidly than the same amount of glucose in other foods. In additions, fats and proteins cause delays in absorption of glucose from carbohydrate eaten at the same time. As well, exercise reduces the need for insulin even when all other factors remain the same, since working muscle has some ability to take up glucose without the help of insulin.
Because of the complex and interacting factors, it is, in principle, impossible to know for certain how much insulin (and which type) is needed to 'cover' a particular meal to achieve a reasonable blood glucose level within an hour or two after eating. Non-diabetics' beta cells routinely and automatically manage this by continual glucose level monitoring and insulin release. All such decisions by a diabetic must be based on experience and training (i.e., at the direction of a physician, PA, or in some places a specialist diabetic educator) and, further, specifically based on the individual experience of the patient. But it is not straightforward and should never be done by habit or routine. With some care however, it can be done reasonably well in clinical practice.
For example, some patients with diabetes require more insulin after drinking skim milk than they do after taking an equivalent amount of fat, protein, carbohydrate, and fluid in some other form. Their particular reaction to skimmed milk is different from other people with diabetes, but the same amount of whole milk is likely to cause a still different reaction even in that person. Whole milk contains considerable fat while skimmed milk has much less. It is a continual balancing act for all people with diabetes, especially for those taking insulin.
Patients with insulin-dependent diabetes require some base level of insulin (basal insulin), as well as short-acting insulin to cover meals (bolus insulin). Maintaining the basal rate and the bolus rate is a continuous balancing act that people with insulin-dependent diabetes must manage each day. This is normally achieved through regular blood tests, although continuous blood sugar testing equipment (Continuous Glucose Monitors or CGMs) are now becoming available.
[edit] Strategies
A long-acting insulin is used to approximate the basal secretion of insulin by the pancreas. NPH/isophane, lente, ultralente, glargine, and detemir may be used for this purpose. The advantage of NPH is its low cost and the fact that you can mix it with short-acting forms of insulin, thereby minimizing the number of injections that must be administered. The disadvantage is that the activity of NPH is less steady and will peak 4–6 hours after administration, and this peak has the potential of causing hypoglycemia. NPH and regular insulin in combination are available as premixed solutions, which can sometimes simplify administration. The theoretical advantage of glargine and detemir is that they only need to be administered once a day, and they also have steady activity, generally without peaks, although in practice, many patients find that neither lasts a full 24 hours. Glargine and detemir are also signifincantly more expensive, and they cannot be mixed with other forms of insulin.
A short-acting insulin is used to simulate the endogenous insulin surge produced in anticipation of eating. Regular insulin, lispro, aspart and glulisine can be used for this purpose. Regular insulin should be given with about a 30 minute lead-time prior to the meal to be maximally effective and to minimize the possibility of hypoglycemia. Lispro, aspart and glulisine are approved for dosage with the first bite of the meal, and may even be effective if given after completing the meal. The short-acting insulin is also used to correct hyperglycemia.
The usual schedule for checking fingerstick blood glucose and administering insulin is before all meals and sometimes also at bedtime. More recent guidelines also call for a check 2 hours after a meal to ensure the meal has been 'covered' effectively. When insulin glargine or insulin detemir is used, it can be administered at any time during the day, provided that it is given at the same time every day.
[edit] Sliding scales
Insulin prescriptions generally specify fixed amounts of long-acting insulin to be given routinely, and fixed amounts of short-acting insulin prior to every meal (the 'sliding scale' approach). However, the amount of short-acting insulin may be varied depending on the patient's preprandial fingerstick glucose, in order to correct pre-existing hyperglycemia. The so-called "sliding-scale" is still widely taught, although it is controversial.[23]
Sample regimen using insulin NPH and regular insulin
| before breakfast | before lunch | before dinner | at bedtime | |
|---|---|---|---|---|
| NPH dose | 12 units | 6 units | ||
| regular insulin dose if fingerstick glucose is (mg/dl) [mmol/L]: |
||||
| 70-100 [3.9-5.5] | 4 units | 4 units | ||
| 101-150 [5.6-8.3] | 5 units | 5 units | ||
| 151-200 [8.4-11.1] | 6 units | 6 units | ||
| 201-250 [11.2-13.9] | 7 units | 7 units | ||
| 251-300 [14.0-16.7] | 8 units | 1 unit | 8 units | 1 unit |
| >300 [>16.7] | 9 units | 2 units | 9 units | 2 units |
Sample regimen using insulin glargine and insulin lispro
insulin glargine 20 units at bedtime
insulin lispro to be given as follows:
| if fingerstick glucose is (mg/dl) [mmol/L]: |
before breakfast | before lunch | before dinner | at bedtime |
|---|---|---|---|---|
| 70-100 [3.9-5.5] | 5 units | 5 units | 5 units | |
| 101-150 [5.6-8.3] | 6 units | 6 units | 6 units | |
| 151-200 [8.4-11.1] | 7 units | 7 units | 7 units | |
| 201-250 [11.2-13.9] | 8 units | 8 units | 8 units | 1 unit |
| 251-300 [14.0-16.7] | 9 units | 9 units | 9 units | 2 units |
| >300 [>16.7] | 10 units | 10 units | 10 units | 3 units |
[edit] Carb counting and DAFNE
A more complicated method that allows greater freedom with meal times and snacks is "carb counting." This approach is taught to diabetic patients in Europe as Dose Adjustment for Normal Eating, or DAFNE. The patient can use his or her total daily dose (TDD) of insulin to estimate how many grams of carbohydrates will be "covered" by 1 unit of insulin, and using this result, the patient can estimate how many units of insulin should be administered depending on the carbohydrate concentration of their meal. For example, if the patient determines that 1 unit of insulin will cover 15 grams of carbohydrates, then they must administer 5 units of insulin before consuming a meal that contains 75 grams of carbohydrates. Some alternative methods also consider the protein content of the meal (since excess dietary protein can be converted to glucose via gluconeogenesis). However, all dosages involve a fair degree of guesswork, and will seldom work consistently from one dosage to the next.
[edit] Abuse
There are reports that some abuse insulin by injecting large doses that lead to hypoglycemic states. This is extremely dangerous. Severe acute or prolonged hypoglycemia can result in brain damage or death.
On July 23, 2004, news reports claimed that a former spouse of a prominent international track athlete said that the ex-spouse had used insulin as a way of 'energizing' the body. There is no evidence to suggest it should act as a performance enhancer in non-diabetics. Poorly controlled diabetics are more prone than others to exhaustion and tiredness, and properly-administered insulin can relieve such symptoms.
"Game of Shadows," by reporters Mark Fainaru-Wada and Lance Williams, includes allegations that Barry Bonds used insulin in the apparent belief that it would increase the effectiveness of the growth hormone he was (also alleged to be) taking. on top of this, non-prescribed insulin is a banned drug at the Olympics and other global competitions.
The use and abuse of exogenous insulin is claimed to be widespread amongst the bodybuilding community. Insulin, human growth hormone (HGH) and insulin-like growth factor 1 (IGF-1) are self-administered by those looking to increase muscle mass beyond the scope offered by anabolic steroids alone. Their rationale is that since insulin and HGH act synergistically to promote growth, and since IGF-1 is a primary mediator of musculoskeletal growth, the 'stacking' of insulin, HGH and IGF-1 should offer a synergistic growth effect on skeletal muscle. This theory has been supported in recent years by top-level bodybuilders whose competition weight is in excess of 50 lb (23 kg) of muscle, larger than that of competitors in the past, and with even lower levels of body fat. There has even been some reaction to the 'freakish' appearance of some of today's top-level professionals.
Bodybuilders are claimed to inject up to 10 i.u. of quick-acting synthetic insulin following meals containing starchy carbohydrates and protein, but little fat, in an attempt to 'force feed' muscle cells with nutrients necessary for growth, whilst preventing growth of adipocytes (ie, fat cells). This may be done up to four times each day, following meals, for a total usage of perhaps 40iu of synthetic insulin per day. However there have been reports of substantially heavier usage, amongst even 'recreational' bodybuilders.
The abuse of exogenous insulin carries with it an attendant risk of hypoglycemic coma and death when the amount used is in excess of that required to handle ingested carbohydrate. Acute risks include brain damage, paralysis, and death. Long-term risks may include development of type 2 diabetes, and potentially a lifetime dependency on exogenous insulin.[citation needed]
[edit] Dietary control of insulin
Many popular weight-loss regimes claim to manipulate weight gain forcing insulin levels (often characterized as insulin overload) by control of carbohydrate intake. Insulin release is controlled by several factors; the carbohydrate stimulus is blood glucose which is not produced by all kinds of carbohydrate. Some types of amino acids also stimulate insulin release. In addition, overly high levels of insulin are seen only in those with pathologies such as Type 2 diabetes mellitus, and only in some of those. The typical person cannot have insulin overload and still have blood glucose levels which do not force symptoms of hypoglycemia. In the non-diabetic, the feedback control mechanism connecting insulin release and blood glucose level is very effective, and it is not possible to adjust it except that blood glucose levels rise slightly during digestion and absorption of glucose. The decrease in blood glucose levels is directly attributable to release of insulin, and that release ceases as blood glucose levels drop. on the other hand, prolonged abnormally low levels of insulin, if it were possible to produce them by control of diet composition and amount, would produce problems with electrolyte balance and with amino acid uptake in cells, among many other effects.
http://www.ncbi.nlm.nih.gov/
http://www.genenames.org/
http://www.genome.ad.jp
'연구하는 인생 > Physiology' 카테고리의 다른 글
| INSULIN - wikipedia (0) | 2008.12.31 |
|---|---|
| Insulin and insulin resistance (0) | 2008.12.31 |
| Diabetic ketoacidosis (DKA) (0) | 2008.12.26 |
| Diabetes mellitus (0) | 2008.12.25 |
| Hypoglycemia 低血糖症 (0) | 2008.12.25 |